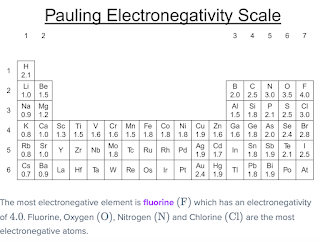
Electronegativity (or rather electron affinity, see this post) of an atom measures the decrease of total energy arising from adding an electron. Pauling suggested a scale to measure electronegativity addressing the following values to the elements in the periodic table:
We see in the 2nd row electronegativity increase from 1.0 for Lithium to 4.0 for Fluorine as the maximum over all elements.onsdag 8 november 2023
ElectroNegativity by RealQM
RealQM gives the following electron affinity values measured in Hartree:
We see that the the 2nd row Pauling scale matches the RealQM values in Hartrees, which makes sense to Pauling's scale.
We note that (i) Helium is missing in the Pauling scale, and (ii) the values for H- differ fundamentally.
The reason the Pauling scale does not take up He is probably the preconceived idea of standard quantum chemistry that He as a noble gas has no incentive at all to catch an electron. RealQM tells a different story, connecting to the previous post showing that He can form a He2 molecule.
On the other hand, RealQM gives H a very small desire to catch an electron, thus supporting the common idea that H acts as an electron donor, in particular when forming the HF molecule by combining with F with maximal electronegativity in an ionic bond.
The Pauling value of 2.0 for H- stands out as strange and in conflict with the idea of ionic bond in HF.
H can form H2 molecule in a covalent bond even with small electronegativity, because no entire capture of an electron is needed, only sharing. RealQM captures the difference in capturing and sharing of electrons, which standard QM does not appear to do.
Prenumerera på:
Kommentarer till inlägget (Atom)




Inga kommentarer:
Skicka en kommentar